Cell Counting & Viability
Cell counts and cell viability are key metrics of every cell-based assay. Analysis of these key metrics are performed daily in thousands of labs around the world for R&D applications and Quality Control in biomanufacturing of cell-based products for gene therapy, stem cell research and cancer cell research. These metrics are important because they provide information about the overall health of the cells while experiments are being carried out on the cells and ensuring quality control during cell manufacturing processes.
Inish Solutions for Cell Counting & Viability
Cellix's Inish Analyser takes the pain away and gives you an automatic cell count and viability measurement in minutes - and that includes sample preparation! Unlike laborious manual cell counting with haemocytometers; image-based analysis or flow cytometry, the Inish Analyser requires no cell staining. This reduces the steps in your workflow, giving you true automated cell counting.

Results
Results are displayed in the form of a scatterplot and in a table.
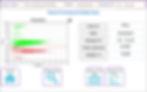



On the scatterplot, there may be up to 3 visible populations depending on your restuls:
-
Live cell population is represented by the green dots.
-
Dead cell population is represented by the red dots.
-
Debris population is represented by the population of dots on the left-hand side of the scatterplot along the Y-axis.

Gating Populations
"Gating” the populations means selecting the population(s) of interest for analysis. For the Cell Counting & Viability Assay, this means determining where the horizontal and vertical lines are positioned:
-
Horizontal Line: Above the line is where the Live cell population is positioned and below the line is where the Dead cell population is positioned.
-
Vertical Line: Left-hand side of this line is where the Debris population is positioned.
The horizontal line is positioned by default at the centre-point, ideally separating the Live cell population from the Dead cell population. However, it may require minor adjustment and you can move the line by placing your finger on the line and sliding it up or down the scatterplot as required. Similarly for the vertical line, you can place your finger on it and slide it left or right. The accompanying table with all the results will be updated accordingly.
Compare with Current Methods
The table below compares Cellix's Inish Analyser which uses impedance spectroscopy; to the most commonly used methods for cell counting and viability which all use cell staining:



Traditional Cell Counting & Viability Analysis: Cell Staining
Traditionally, cell counting and viability analysis is performed by staining the cells. DAPI (4', 6-diamido-2-phenylindole) and Hoechst are commonly used stains or dyes for cell counting. They stain the nucleus of both live and dead cells and appear blue in colour.
A common example of cell viability stains include:
Live cells stained with a green stain (Calcein AM) and
Dead cells with a red stain (Propidium Iodide)
There are now a range of cell viability stains available, including Trypan Blue for dead cells (blue stain) and Erythrosine B for dead cells (pink stain).

Problems with Cell staining:
-
Time consuming: Manual step of cell staining. Depending on the cell staining kit or dyes used, it can take up to one hour to complete the sample preparation of staining the cells.
-
Stains affect cell functionality: The addition of a stain itself can affect cell functionality.
Haemocytometers / Hemocytometers
Haemocytometers are counting chambers and they have been used by cell biologists for >100 years. Despite the recent introduction of semi-automated methods such as those described here (i.e. image analysis and flow cytometry); haemocytometers are still a mainstay of many cell biology laboratories worldwide. But recurring challenges with haemocytometer-based cell counting is resulting in the gradual replacement of these methods.
Challenges of Haemocytometer Cell Counting:
-
Human Error: Manual counting means that this is inevitable at multiple stages of the process including mixing, handling and dilution.
-
Time consuming: Due to the manual nature of the process, it is not suited for large scale analyses or processes which require a high throughput cell analysis.
-
Poor reproducibility: As multiple counts are required to ensure consistency, it often leads to poor reproducibility especially across user-to-user testing of a cell sample.
-
Material variation: As this is a DIY method, each researcher will use different buffers, different pipettes and these differences can result in further unwanted errors.

Image-based Analysis
As computer processing capabilities have increased in recent years, so too has the rise of computer-based image cytometry. With more robust machine learning and image analysis algorithms, computers can now quantitatively analyse cellular images detecting features that may not be detected by the human eye. Instruments relying on this method include a digital camera (to take the image of the cells) and a computer program to analyse the image. The camera must take a good quality image with "cells-in-focus" - good quality images are easier for the computer algorithm to analyse. Thus, these devices often have a manual focus or auto-focus setting - this ensures the cells are in focus before images are taken. To ensure correct presentation of the cell sample in front of the camera, these instruments typically use specialist slides or cartridges which are specific to the instrument.

While image-based analysis has gained traction in recent years, there are a number of disadvantages to this method:
Challenges of Image-based Analysis:
-
Manual adjustments are required: Adjustments in staining are often required to obtain a better resolution of cells from the background image. High Trypan blue concentration can lead to background noise and interfere with the ability of the instrument to correctly analyse and distinguish cells.
-
Semi-automatic: The user must stain the cells before loading the cell sample in the slide or cartridge.
-
High cost per assay: As each instrument has a chamber, slide or cartridge which is specific to that instrument, end-users are tied to the high cost of consumables for every assay.
-
Large volumes of imaging data: Advances in image-based analysis include image-based cytometers (high-resolution imaging in microscopy of single cells in flowing environments). While popular in recent years, these instruments face computational challenges in handling large volumes of image data that need to be processed which limits their use for clinical applications.
Flow Cytometry
Flow cytometry method involves flowing cells, one at a time, past a light source (e.g. laser) and a detector. As the cell passes the light source, the scattered light is collected by the detector and analysed. Cells are labeled with fluorescent stains which result in absorption and emission of light at different wavelengths. Depending on the wavelength of the emitted light, different characteristics of the labelled cell can be measured.
Challenges of Flow Cytometry:
-
Complex instrumentation: Most flow cytometers are relatively complex instruments which are overkill for applications such as simple cell counting and viability analysis. While there are more bench-top flow cytomters on the market now, many are still confined to flow cytometry core facilities operated by technicians.
-
High instrument cost: As flow cytometers are capable of multi-parametric analysis (including cellular component analysis such as organelles, nuclei, DNA, RNA, chromosomes, cytokines, hormones and protein content); these instruments command a much higher price compared to simplified image-based analysis instruments.

Why Measure Cell Counts & Viability?
Cell counts are particularly important in cases where cells are being cultured for another purpose e.g. further experimentation or where the cells themselves are the product, such as cell therapy. Depending on the experiments or cell manufacturing processes to which the cells are exposed, cell viability may be affected. This means that cell viability is often checked at various points throughout an experiment or process.
Factors which affect cell viability include:
-
Pressure: Dispensing of cells from cell sorters results in a large drop in pressure (often referred to as "explosive decompression") which can affect cell viability.
-
Temperature: Experiments which can result in increased temperatures (e.g. certain biomaterials required for 3D cell printing) need to be planned carefully to avoid exposing cells to high temperatures.
-
Availability of sufficient nutrients: Experiments which deprive the cells of access to nutrients for prolonged periods of time can adversely affect cellular health.
-
Exposure to toxic substances: A critical test which all substances, including drugs, must pass before being approved. As a result, toxicity screening ("tox screens") are a huge area of study whereby cells are purposefully exposed to different concentrations of a substance to determine their level by which cell viability is affected. Important for new drug therapies and also to determine safe usage of every-day chemicals in the home.
-
Shear forces: Forces induced by the walls of flow cells or the walls of a needle - these forces tend to have less of an effect compared with pressure.










.jpg)
.jpg)

.jpg)
.jpg)